Intro to EEG Data Analysis
Background
Two years ago I have been working on EEG data (found at Kaggle1) from an object recognition task experiment that was performed to investigate the genetic predisposition to alcoholism2. My analysis was a pure mess since I didn’t know a lot about EEG data processing/analysis. Time has passed, I still don’t know much, but after finishing Mike X Cohen’s course “Complete neural signal processing and analysis: Zero to hero” on Udemy3 (which I cannot recommend highly enough), I decided to get more practice with the help of publicly available data from Cavanagh et al. (2019)4. I used MNE Python package5 for this and tried to do it in a bit “tutorial” style for two reasons. First, it will be easier for me to remember after some time what the hell was I doing here. Second, I hope that other people who also start their journey in the EEG analysis can benefit from this.
Disclaimer
This analysis was made for educational purposes in order to get an idea of how to work with the EEG data. I was not going to make any inference/conclusions about subjects or conditions (even if I did, they should not be taken seriously).
Overview of the Experiment

The original paper was published in Computational Psychiatry open source journal (the MIT Press)6 in 2018. One of the aims of the experiment was to investigate the association of EEG responses to reward and punishment among two groups, the control group (CTL) and the high depressive symptomatology group (DEP). Participants performed a probabilistic learning task, where a combination of two different images from the set was shown. Each image had its own probabilistic chance of being correct. The task was divided into training (participants were expected to learn the probability of being correct for each stimulus) and testing phases (experiment itself).
Participants:
- CTL: N = 75 (50 females)
- DEP: N = 46 (34 females)
- An average age among all participants was 18 years.
Preprocessing
Data Import
For simplicity, I took just two data files, one subject from the DEP group (ID: 561), and one subject from the CTL group (ID: 507).
1import mne
2import pickle
3import numpy as np
4import pandas as pd
5from scipy.io import loadmat
6
7%matplotlib qt
1# loading raw matlab files
2CTL = loadmat('depression_data/task/507.mat')
3DEP = loadmat('depression_data/task/561.mat')
4
5EEG = dict()
6# data comes in a funny structure, so you have to dig deep with indexes
7# to extract it. `squeeze_me` argument doesn't look to help in such case
8EEG['timevec'] = CTL['EEG']['times'][0][0][0]
9EEG['srate'] = int(CTL['EEG']['srate']) # 1 sample/2 ms
10
11# get the channel labels in a bit sophisticated way
12EEG['chanlabels'] = list(np.hstack(CTL['EEG']['chanlocs'][0][0]['labels'][0]))
13# mne package requires channel labels to be in a particular way,
14# so here I just change the cases for some channels
15for i in range(len(EEG['chanlabels'])):
16 EEG['chanlabels'][i] = EEG['chanlabels'][i].replace('Z', 'z')
17 EEG['chanlabels'][i] = EEG['chanlabels'][i].replace('FP', 'Fp')
18
19info = mne.create_info(
20 ch_names=EEG['chanlabels'],
21 sfreq=EEG['srate'],
22 ch_types='eeg')
23
24# convert raw data into mne object
25# also initial values were in microvolts, so convert them into volts
26EEG['CTL_data'] = mne.io.RawArray(CTL['EEG']['data'][0][0]*10**(-6), info)
27EEG['DEP_data'] = mne.io.RawArray(DEP['EEG']['data'][0][0]*10**(-6), info)
Creating RawArray with float64 data, n_channels=66, n_times=356405
Range : 0 ... 356404 = 0.000 ... 712.808 secs
Ready.
Creating RawArray with float64 data, n_channels=66, n_times=575973
Range : 0 ... 575972 = 0.000 ... 1151.944 secs
Ready.
Data was recorded at the 500 Hz rate. In other words, recordings were done every seconds. Total duration of the recording for the CTL subject is 712.808 seconds, which is approximately 11-12 minutes. Total duration of the recording for the DEP subject is 1151.944 seconds, which is approximately 19 minutes.
Recordings were measured from 66 channels, including two electrooculography (EOG) electrodes placed near the eye (HEOG, VEOG), two electrodes on mastoid processes (M1, M2) and two cerebellar electrodes (CB1, CB2).
1biosemi_montage = mne.channels.make_standard_montage('biosemi64')
2EEG['CTL_data'].set_montage(biosemi_montage, on_missing='ignore')
3EEG['DEP_data'].set_montage(biosemi_montage, on_missing='ignore')
Some of the channels (M1, M2, PO5, PO6, CB1, CB2, HEOG, VEOG) were not recognized by the Biosemi 64 montage. It’s “okay” since I would drop most of them anyway (leaving 60 electrodes). HEOG and VEOG channels were used to detect eye blinks and to compare with ICA components. M1 and M2 channels were used for reference. Cannot say much about PO5 and PO6 channels. In the original paper, they also reported that they used 60 channels plus two mastoids for the preprocessing stage.
1# referencing the data
2# channel_i = channel_i - mean(channel_M1, channel_M2)
3EEG['CTL_data'] = EEG['CTL_data'].set_eeg_reference(ref_channels=['M1', 'M2'])
4EEG['DEP_data'] = EEG['DEP_data'].set_eeg_reference(ref_channels=['M1', 'M2'])
EEG channel type selected for re-referencing
Applying a custom EEG reference.
EEG channel type selected for re-referencing
Applying a custom EEG reference.
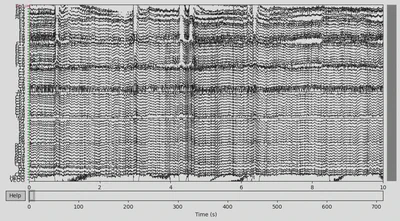
EEG data of the CTL subject:
1EEG['CTL_data'].plot(n_channels=len(EEG['CTL_data'].ch_names))
EEG data of the DEP subject:
1EEG['DEP_data'].plot(n_channels=len(EEG['DEP_data'].ch_names))
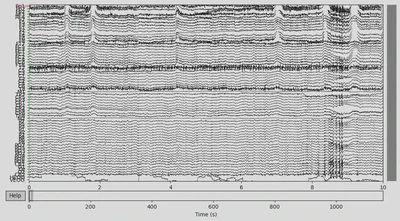
There was some funny business (voltage several orders higher than the rest of the signal) happening at the very of the recording, which I have removed.
1EEG['CTL_data'].crop(tmax=700)
2EEG['DEP_data'].crop(tmax=1140);
Bad Channels/Trials
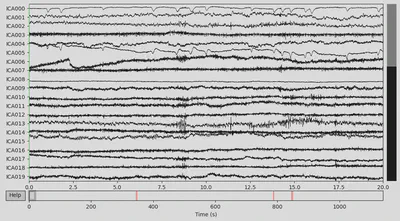
All the channels for CTL subject looked fine to me. However, for the DEP subject F2 channel looked a bit suspicious (only high-frequency fluctuations), so I marked it as “bad”.
The next step was to extract events data from the raw object and add it to the EEG data. There were lots of different event IDs, but I just used two specific ones: 94, meaning correct trial, and 104, meaning incorrect trial.
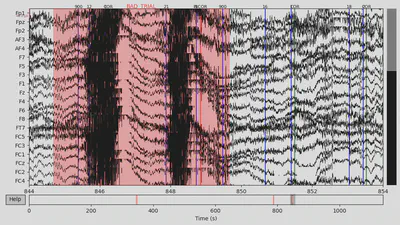
Then I annotated “bad” time spans. I paid attention to those that overlap with 94/104 events with the +/- 2 seconds interval since this time interval would be used for making epochs and all other epochs would be lost anyway. There were several time spans for both subjects that looked “bad” for me. An example can be seen below on the plot, with the suspiciously high-frequency signal.
1# extracting events info from the raw file
2for data in [CTL, DEP]:
3 event_df = pd.DataFrame(data['EEG']['event'][0][0][0])
4 # fun with indexes again
5 event_df['type'] = event_df['type'].apply(lambda x: x[0])
6 for col in ['latency', 'urevent']:
7 event_df[col] = event_df[col].apply(lambda x: x[0][0])
8
9 # convert strings to integers
10 event_df['type'] = event_df['type'].apply(lambda x: 900 if x == 'keyboard0' else (
11 1 if x == 'keypad1' else (
12 2 if x == 'keypad2' else (
13 999 if x == 'boundary' else int(x)))))
14
15 # mne requires events data to be in numpy array with three columns:
16 # time of occurence; second column for whatever reason which is ignored anyway; event id
17 events = np.array(event_df[['latency', 'type']])
18 events = np.insert(events, 1, values=0, axis=1)
19
20 if data == CTL:
21 EEG['CTL_events'] = events
22 else:
23 EEG['DEP_events'] = events
24
25# just a fancy way to add colors and labels to the plot
26event_color = dict(zip(event_df['type'].unique(), ['b']*len(event_df['type'].unique())))
27event_color[94] = 'g'
28event_color[104] = 'r'
29
30event_id = {'COR': 94, 'INCOR': 104}
31
32del event_df, events, CTL, DEP
1EEG['CTL_data'].plot(events=EEG['CTL_events'], start=182, event_id=event_id, event_color=event_color)
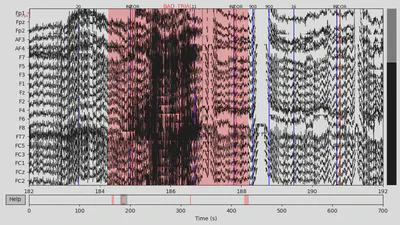
1EEG['DEP_data'].plot(events=EEG['DEP_events'], start=844, event_id=event_id, event_color=event_color)
1# marked bad channel
2EEG['DEP_data'].info['bads']
['F2']
Artifacts Removal
The next step was to remove eye blinks from the data. HEOG and VEOG electrodes were put around an eye to measure the horizontal and vertical eye movements respectively and they could be used for references. I applied an Independent Component Analysis (ICA)7 to separate the signal sources.
1# drop the redundant channels
2EEG['CTL_data'].drop_channels(ch_names=['HEOG', 'VEOG', 'CB1', 'CB2', 'M1', 'M2', 'PO5', 'PO6'])
3EEG['DEP_data'].drop_channels(ch_names=['HEOG', 'VEOG', 'CB1', 'CB2', 'M1', 'M2', 'PO5', 'PO6'] + EEG['DEP_data'].info['bads'])
CTL Group
1# mne tutorial suggests to filter the data from low frequencies (keep 1Hz+)
2# since they can affect ICA result
3filt_raw = EEG['CTL_data'].copy()
4filt_raw.load_data().filter(l_freq=1., h_freq=None)
Filtering raw data in 1 contiguous segment
Setting up high-pass filter at 1 Hz
FIR filter parameters
---------------------
Designing a one-pass, zero-phase, non-causal highpass filter:
- Windowed time-domain design (firwin) method
- Hamming window with 0.0194 passband ripple and 53 dB stopband attenuation
- Lower passband edge: 1.00
- Lower transition bandwidth: 1.00 Hz (-6 dB cutoff frequency: 0.50 Hz)
- Filter length: 1651 samples (3.302 sec)
1ica = mne.preprocessing.ICA()
2ica.fit(filt_raw)
Fitting ICA to data using 58 channels (please be patient, this may take a while)
Inferring max_pca_components from picks
Omitting 8222 of 350001 (2.35%) samples, retaining 341779 (97.65%) samples.
Selecting all PCA components: 58 components
Fitting ICA took 80.6s.
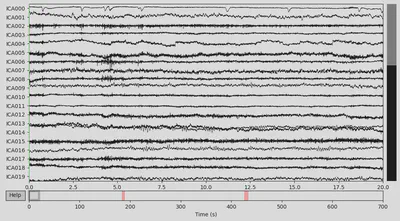
1ica.plot_sources(EEG['CTL_data'])
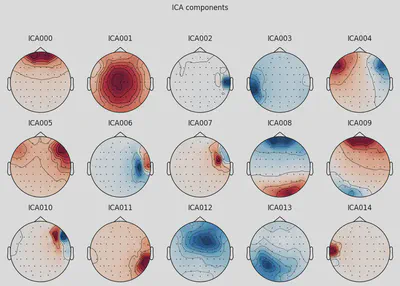
1ica.plot_components(picks=range(15))
1# make a plot of signal without the first component
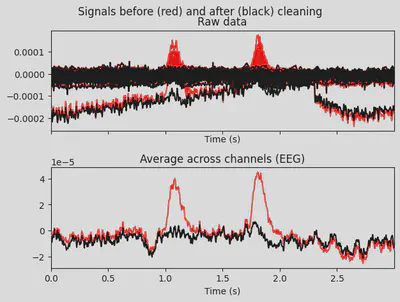
2ica.plot_overlay(EEG['CTL_data'], exclude=[0], picks='eeg')
Transforming to ICA space (58 components)
Zeroing out 1 ICA component
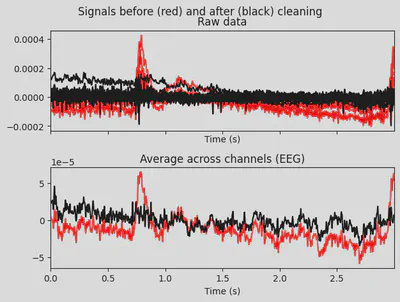
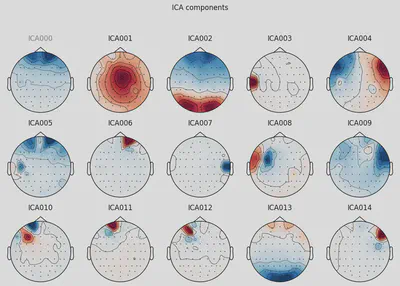
THe first (ICA000) component was clearly an eye blink. The signal was stable for the whole time with some occasional drops caused by events at the front of the head.
Some other components, like ICA002 or ICA014, also looked weird for me, but that’s something for me to work on.
1ica.exclude = [0] # exclude just first (ICA000) component
2ica.apply(EEG['CTL_data'])
3
4del ica, filt_raw
Transforming to ICA space (58 components)
Zeroing out 1 ICA component
DEP Group
1filt_raw = EEG['DEP_data'].copy()
2filt_raw.load_data().filter(l_freq=1., h_freq=None)
Filtering raw data in 1 contiguous segment
Setting up high-pass filter at 1 Hz
FIR filter parameters
---------------------
Designing a one-pass, zero-phase, non-causal highpass filter:
- Windowed time-domain design (firwin) method
- Hamming window with 0.0194 passband ripple and 53 dB stopband attenuation
- Lower passband edge: 1.00
- Lower transition bandwidth: 1.00 Hz (-6 dB cutoff frequency: 0.50 Hz)
- Filter length: 1651 samples (3.302 sec)
1ica = mne.preprocessing.ICA()
2ica.fit(filt_raw)
Fitting ICA to data using 57 channels (please be patient, this may take a while)
Inferring max_pca_components from picks
Omitting 5585 of 570001 (0.98%) samples, retaining 564416 (99.02%) samples.
Selecting all PCA components: 57 components
Fitting ICA took 141.5s.
1ica.plot_sources(EEG['DEP_data'])
1ica.plot_components(picks=range(15))
1ica.plot_overlay(EEG['DEP_data'], exclude=[0], picks='eeg')
Transforming to ICA space (57 components)
Zeroing out 1 ICA component
1ica.exclude = [0]
2ica.apply(EEG['DEP_data'])
3
4del ica, filt_raw
Transforming to ICA space (57 components)
Zeroing out 1 ICA component
DEP subject got even more “suspicious” components, which I ignored for now.
1# at this stage I used to save the EEG locally to avoid repeating the same steps
2# every time I rerun the notebook
3
4# with open('EEG.pickle', 'wb') as f:
5# pickle.dump(EEG, f)
6
7# with open('EEG.pickle', 'rb') as f:
8# EEG = pickle.load(f)
Event-related potential
The final step (for this part) was to get the Event-Related Potential (ERP)8 for both conditions: correct and incorrect, meaning that there were 4 ERPs: DEP&COR, DEP&INCOR, CTL&COR, CTL&INCOR.
Epochs
First signal had to be split into epochs according to the events (94/104) times. Time window is +/- 2 seconds around the feedback onset. Then signal was baseline corrected to the average signal from -0.2 to 0 seconds prefeedback. Bandpass filter (0.5-20 Hz) was applied.
1for g in ['DEP', 'CTL']:
2 for o in ['correct', 'incorrect']:
3 if o == 'correct':
4 eid = 94
5 else:
6 eid = 104
7
8 # copy of the signal to apply the bandpass filter on
9 filt_raw = EEG[f'{g}_data'].copy()
10 filt_raw.load_data().filter(l_freq=0.5, h_freq=20, verbose=False)
11
12 EEG[f'{g}_epochs_{o}'] = mne.Epochs(
13 raw=filt_raw,
14 events=EEG[f'{g}_events'],
15 event_id=eid,
16 tmin=-2,
17 tmax=2,
18 baseline=(-0.2, 0))
19
20 # drop bad time spans
21 EEG[f'{g}_epochs_{o}'].drop_bad()
22 # get ERP
23 EEG[f'{g}_erp_{o}'] = EEG[f'{g}_epochs_{o}'].average()
24 EEG[f'{g}_erp_{o}'].comment = f"{g}&{o}"
Total trials for each case:
- CTL & correct: 72 total events, 7 bad events, final 55 events.
- CTL & incorrect: 48 total events, 3 bad events, final 45 events.
- DEP & correct: 169 total events, 5 bad events, final 164 events.
- DEP & incorrect: 125 total events, 4 bad events, final 121 events.
Resulted ERPs
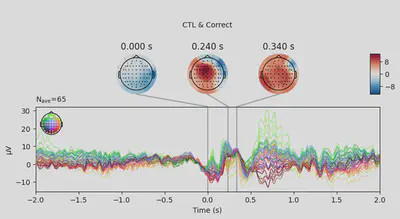
1EEG['CTL_erp_correct'].plot_joint(times=[0, 0.24, 0.34], title='CTL & Correct')
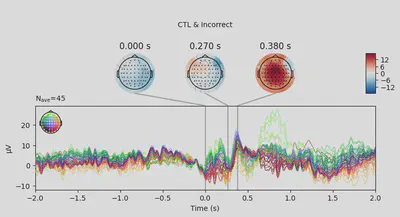
1EEG['CTL_erp_incorrect'].plot_joint(times=[0, 0.27, 0.38], title='CTL & Incorrect')
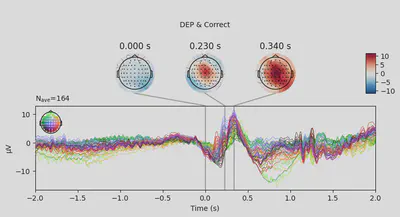
1EEG['DEP_erp_correct'].plot_joint(times=[0, 0.23, 0.34], title='DEP & Correct')
1EEG['DEP_erp_incorrect'].plot_joint(times=[0, 0.27, 0.38], title='DEP & Incorrect')
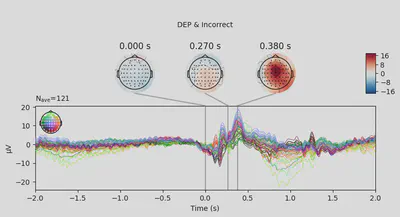
In most of the cases, picks in poststimulus response were caused by the increased activity around the Fz-FCz-Cz areas. Below is the comparing ERP signal at the FCz channel for CTL and DEP group for each outcome.
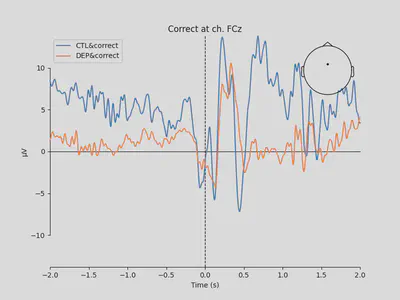
1mne.viz.plot_compare_evokeds(
2 [EEG['CTL_erp_correct'].copy().pick_channels(['FCz']),
3 EEG['DEP_erp_correct'].copy().pick_channels(['FCz'])],
4 title='Correct at ch. FCz')
1mne.viz.plot_compare_evokeds(
2 [EEG['CTL_erp_incorrect'].copy().pick_channels(['FCz']),
3 EEG['DEP_erp_incorrect'].copy().pick_channels(['FCz'])],
4 title='Incorrect at ch. FCz')
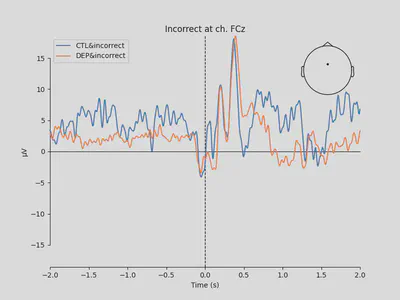
The plots looked somewhat similar to the plot in the original paper (Figure 3). Prominent N2-P3 peaks for incorrect trials and two peaks at approximately the same level for correct trials.
Summary
This was it for now. I learned how to perform first preprocessing steps on EEG data (referencing, eye blinks removal, baseline correction, epochs splitting). Some parts like detecting bad channels/trials and separating noise from the signal using ICA need more experience and practice from me.
In the next part I am going to perform a time-frequency analysis for both subjects.
References
Ruslan Klymentiev (2019, January 8). EEG Data Analysis. Kaggle. https://www.kaggle.com/code/ruslankl/eeg-data-analysis/ ↩︎
Complete neural signal processing and analysis: Zero to hero | Udemy ↩︎
Cavanagh, J. F., Bismark, A. W., Frank, M. J., & Allen, J. J. B. (2019). Multiple Dissociations Between Comorbid Depression and Anxiety on Reward and Punishment Processing: Evidence From Computationally Informed EEG. Computational Psychiatry, 3, 1–17. DOI: http://doi.org/10.1162/CPSY_a_00024 ↩︎
Independent Component Analysis - an overview | ScienceDirect Topics ScienceDirect ↩︎
Event-Related Potential - an overview | ScienceDirect Topics ScienceDirect ↩︎